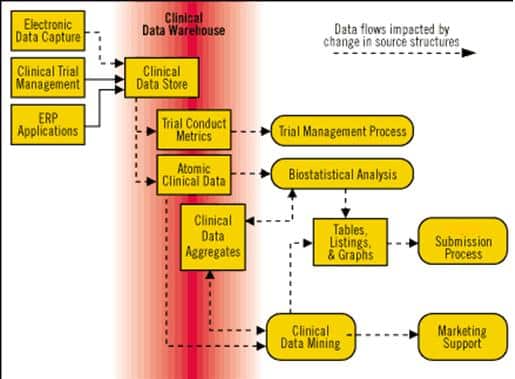
Data Management
Proper handling of clinical data is a predictor of success in the clinical studies. Farzan Clinical Research will ensure that data management of your study is performed to the highest standards, in compliance with GCDMP guideline, as Society for clinical data management (SCDM) recommends, and also according to Farzan Clinical Research SOPs.
We use an up-to-date best in class clinical research software (Faradata). With this software you would be able to both handle your data and the related database in a single study and manage different studies. It also gives you the benefits of getting SPSS file export and therefore statistical analysis of the study would be as easy as possible.
The important characteristic of this software is to be web-based that allows you to simultaneously manage different sites and different multi-center studies from your office or even at home!
Safety data management and reporting is also accomplished according to E2A, E2B, E2C, E2D, E2E ICH guidelines.
Our services for data management contain:
- Creating database at your site or ours and according to your specifications.
- Detailed case report form (CRF) Design and Review.
- Double-Data Entry.
- Electronic Data Capture projects.
- Data listings or reporting to your requirements.
- Comprehensive Data Validation.
- Data Quality Assurance.
- Dictionary Preferred-Term Coding.
- Data Management Training and Consultancy.
- Data Analysis.
- Data Management Reporting.
- Data Review Meeting.
- Database Locks with Associated Documentation.
- Database transfer in Sponsor defined formats with detailed documentation.
Target Group
The potential clients of Farzan Clinical Research Data management services are:
- Pharmaceutical and Medical Device Industry
- Research Centers and Universities
- Medical Societies
- Clinical Investigators
- Clinicians and clinics
